Leaky gut syndrome, also known as gut leakiness or gut permeability, is a phenomenon that is increasingly being studied in modern science. It is a condition in which the gut wall is more permeable than normal (1, 10). This allows various substances, such as bacteria, toxins, and nutrients, to pass through the gut barrier and enter the bloodstream (1, 6). Gut permeability specifically affects the mucosal barrier that lines the intestines (1, 10).
Research shows that diet plays a key role in gut barrier health, affecting the intestine’s structure (mucosal barrier thickness) and function (epithelial cell roles) (3). Let’s explore how diet influences gut leakiness and its possible clinical implications.
1. Physiology of the gut barrier
What is the gut barrier?
The gut barrier consists of three layers: a mucosal barrier, a physical barrier, and an immune barrier. The physical barrier is consistent throughout the intestines and is made of epithelial cells (1, 2, 6). These cells are connected by several structures, including tight junctions (1, 2, 3, 5, 6, 10). Tight junctions are essential components of the gut barrier that determine what can and cannot pass through. For instance, certain ions, water, and sugars can pass through, but pathogenic bacteria and toxic compounds cannot (1, 2, 6, 9).
The mucosal barrier varies in composition at different points in the intestines (1, 2, 3). In the small intestine, the mucus forms a thin, discontinuous layer that facilitates nutrient absorption, while in the large intestine, the mucus has two layers (1, 3). These layers create a protective physical separation between the body and the gut’s contents and prevent harmful bacteria from adhering (1, 3, 6).
The immune barrier consists of protective cells and molecules, such as defensins, lymphocytes, and macrophages, which manage immunity (1, 2).
Leaky gut syndrome occurs when the gut barrier becomes hyperpermeable (6), allowing substances such as bacteria, toxins, and nutrients to pass through the gut wall and enter the circulatory system (1, 6, 10). These substances can trigger various immune and inflammatory responses (1, 8). Chronic hyperpermeability can alter the function of tight junctions and increase gut leakiness (10).
What role does the gut microbiota play?
Studies report that the gut microbiota plays a key role in maintaining the integrity of the gut wall (1, 6, 10). Several of its functions are linked to regulating the gut barrier. These include controlling the proliferation of pathogenic bacteria, stimulating the immune system, and synthesizing short-chain fatty acids (SCFAs) (1, 6, 10). An imbalance in the gut microbiota, known as dysbiosis, can alter the tight junctions, allowing pathogens and toxins, such as lipopolysaccharides (LPSs), to enter (1, 6, 10).
How can gut permeability be assessed?
In trials, gut permeability can be measured by checking various blood markers of barrier membrane protein and tight junction expression (1, 2, 3, 4, 10). Examples of these markers include zonulin, occludin, and claudin (1, 3, 4). Systemically circulating endotoxins, or LPSs, can also be measured as they reflect potential damage to the gut wall (3). Another method of assessment is to measure the ability of certain ingested sugars, such as lactulose and mannitol, to permeate the mucosal barrier (1). Although the degree of permeability can be determined physically through a biopsy, this isn’t often done because the procedure is invasive. Instead, the condition of the gut wall is mainly assessed using experience and clinical judgment. Gut permeability is generally considered a symptom of an underlying problem (1, 4, 10).
What factors influence gut barrier health?
There are several factors that can disrupt the gut barrier. In addition to diet and gut bacteria, factors such as antibiotic use, age, genes, H. pylori infections, medication, hormones, inflammation, physical and psychological stress, and the intensity of physical activity can influence gut barrier integrity (1, 2, 6).
2. Nutritional modulation mechanism
The state of the gut barrier is influenced by food. SCFAs such as acetate, butyrate, and propionate are key elements of promoting a healthy gut barrier (2, 3). They are synthesized by gut bacteria from the fermentation of certain nutrients, including fibre and prebiotics (1, 2, 5, 6). Butyrate promotes gut barrier integrity by synthesizing molecules that strengthen the tight junctions and mucus layers (1, 2, 3, 5, 6, 9). Many studies report that butyrate and propionate have a “tightening” effect on the tight junctions, reducing permeability (2, 5). SCFAs (mainly butyrate) also serve as energy sources for colonocytes (1, 3). Deficiencies in SCFAs and fibre can compromise epithelial cell functioning in the physical barrier by increasing gut permeability and altering the mucus (1).
3. Key nutrients and bioactive compounds
We’ve established that diet influences gut wall health. Let’s take a closer look at the key nutrients involved and their impact.
Fats
Studies report that a high-fat diet can increase gut leakiness by modulating the expression and distribution of tight junctions (3). Mechanisms that explain the negative effects of fat on the gut wall include reducing bacteria that strengthen its integrity, increasing bacteria that weaken it, and activating inflammatory cascades (3).
However, as with many health conditions, the impact varies depending on the type of fat. Saturated fats have been associated with increased gut permeability due to their effect on bacteria (2, 6). Conversely, the fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been shown to support gut barrier integrity (2, 5, 6).
Fibre and prebiotics
As mentioned above, fibre and prebiotics are fermented by gut bacteria, promoting the synthesis of SCFAs. SCFAs play important roles in the gut barrier, including regulating the mucosal immune barrier (1, 3). Similarly, fibre can positively alter the gut mucosal barrier (3). Dietary fibre is found in whole grains, legumes, fruits, vegetables, nuts, and seeds. The main sources of prebiotics are beets, inulin, garlic, onions, bananas, leeks, and berries (2, 3, 6).
For more details, read our blog articles on the gut microbiota and prebiotics.
Added sugar
A low-sugar diet is beneficial for maintaining healthy tight junctions (1, 2, 7). Several studies have examined the role of different sugars (fructose, glucose, and sucrose) in gut permeability (1, 7). These types of added sugar appear to increase gut leakiness, inflammatory cytokines, and tight junction dysfunction (1, 2, 7, 9). Some studies have found that fructose intake can increase gut permeability and promote the release inflammatory factors into the liver, thereby increasing liver and systemic inflammation (2, 7, 9). However, it should be noted that these studies are preliminary. Reducing added sugar and high-carbohydrate foods overall appears important for maintaining a healthy gut barrier.
Alcohol
High alcohol consumption is associated with an increased risk of developing leaky gut syndrome (1, 3, 5, 6). Alcohol consumption can inhibit beneficial bacteria and impair gut barrier function and SCFA production. It can also promote dysbiosis and alter the composition of the mucosal microbiota (1, 3, 5). As with other health conditions, limiting alcohol consumption or abstaining altogether is beneficial for gut health.
Additives and emulsifiers
Food additives have been shown to increase gut permeability by interfering with tight junctions and promoting the passage of immunogenic antigens (1, 6). Emulsifiers have been associated with reduced bacterial diversity, increased mucosal inflammation, and bacterial translocation (2). For instance, carboxymethylcellulose (CMC) and polysorbate 80 (P80) have been shown to thin the mucus layer (2). However, most trials were conducted on animals, with few conducted on humans to confirm these effects. Further research is needed.
Below is a diagram of the nutrients that influence the gut barrier.
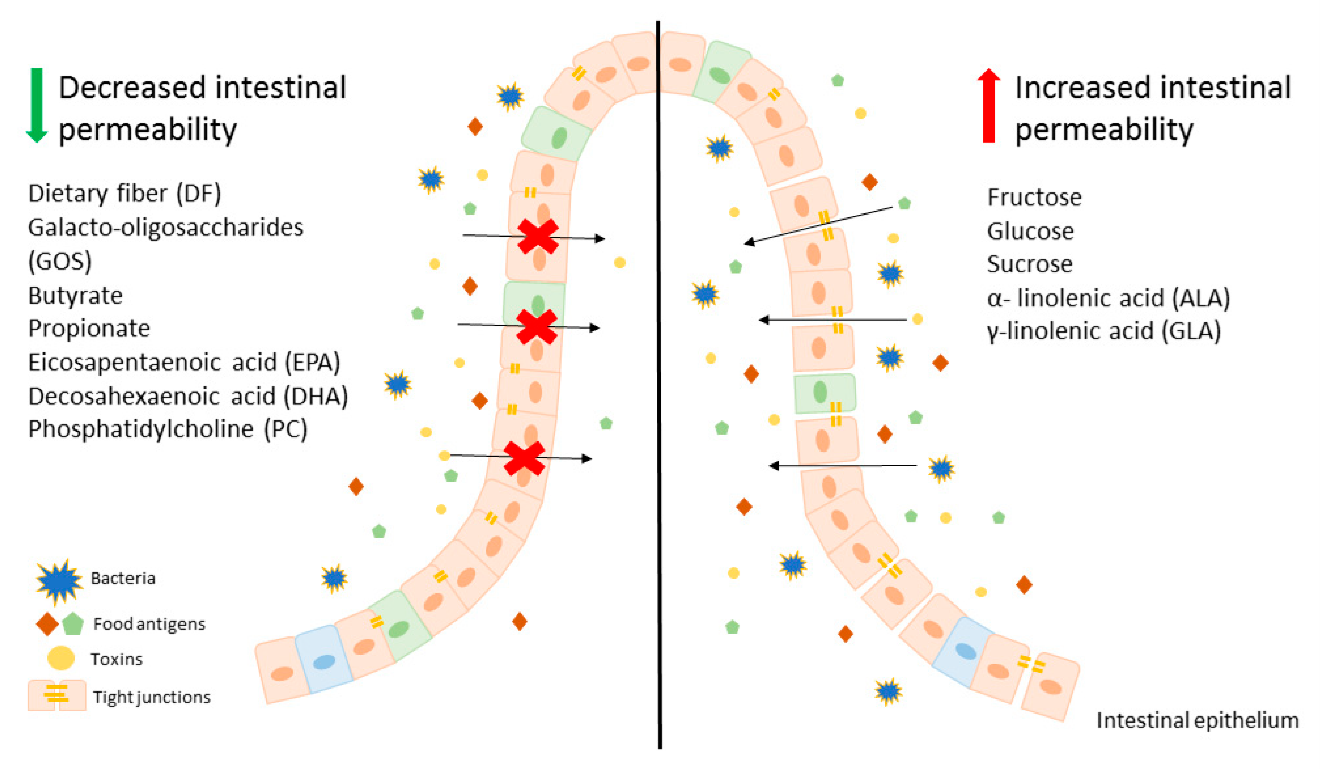

Source: “An overview on the effect of various components of diet on intestinal epithelium permeability.” Binienda et al. (2020) (2)
4. Clinical implications
In addition to clarifying the impact of diet on gut permeability, studies have examined the health implications of leaky gut. Diverse systemic consequences associated with gut barrier dysfunction have been reported, including increased inflammation, immune response activation, oxidative stress, and decreased insulin sensitivity, which affect tissues and organs such as the liver, adipose tissue, muscles, and brain (3).
Below is a list of conditions associated with gut hyperpermeability and their potential underlying mechanisms (1, 2, 3, 4, 6, 7, 8, 9, 10):
- Irritable bowel syndrome (IBS): lower zonulin and occludin levels in gut tissue, dysbiosis, and bacterial translocation
- Small intestinal bacterial overgrowth (SIBO): alteration of the gut microbiota
- Celiac disease: increased gut inflammation and alteration of tight junctions when gluten is ingested
- Obesity: changes in gut bacteria composition, increased plasma endotoxins, and reduced SCFAs
- Inflammatory bowel disease (IBD), such as Crohn’s disease and ulcerative colitis: higher levels of inflammation and altered tight junctions
- Rheumatoid arthritis: inflammatory response and alterations in the gut microbiota
- Cardiovascular disease: increased endotoxins and inflammatory markers and changes in the gut microbiota
- Type 1 and type 2 diabetes: altered gut barrier function and bacterial translocation
- Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD): dysfunction of the gut-liver axis, dysbiosis, altered bacteria, and inflammatory response
Current scientific research reports associations between gut barrier disruption and these pathologies (1, 5). However, there is no proven causal relationship, and further studies are needed to determine the order of disruption (1, 5). Do these diseases cause leaky gut, or does leaky gut cause these diseases? The answer is not yet known.
Although there are general recommendations for maintaining a healthy gut wall, it’s important to take a personalized approach. This is because existing diseases, lifestyle habits, digestive symptoms, and bacterial signatures are all unique to each individual. Personalized nutritional assessments and management plans are essential for sustaining dietary changes and optimizing digestive health.
Conclusion
In conclusion, gut mucosal health can be influenced by many factors. There is an increasing amount of scientific data on the subject, which is promising. Adopting a healthy lifestyle will, of course, have a positive effect on the gut mucosa. Consuming dietary fibre and omega-3 fatty acids while reducing saturated fat, added sugar, alcohol, and processed food intake will help maintain a healthy gut wall.
This article provides general information only and is not intended to replace the advice or care of a healthcare professional. The effects of the products described in this text may vary from person to person; some of them may be contraindicated for you and may interact with your medications if you are taking any. If you are being treated for a health problem, consult a healthcare professional before incorporating them into your diet.
References:
[1] Aleman et al. (2023) Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules. Jan 7;28(2):619. https://pubmed.ncbi.nlm.nih.gov/36677677/
[2] Binienda et al. (2020) Dietary Carbohydrates and Lipids in the Pathogenesis of Leaky Gut Syndrome: An Overview. Int J Mol Sci. Nov 8;21(21):8368. https://pubmed.ncbi.nlm.nih.gov/33171587/
[3] Camilleri and Vella (2022) What to do about the leaky gut. Gut. Feb;71(2):424-435. https://pubmed.ncbi.nlm.nih.gov/34509978/
[4] Chae et al. (2024) Diet-Induced Gut Dysbiosis and Leaky Gut Syndrome. J Microbiol Biotechnol. Apr 28;34(4):747-756. https://pubmed.ncbi.nlm.nih.gov/38321650/
[5] De Santis et al. (2015) Nutritional Keys for Intestinal Barrier Modulation. Front Immunol. Dec 7;6:612. https://pubmed.ncbi.nlm.nih.gov/26697008/
[6] Di Vincenzo et al. (2024) Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. Mar;19(2):275-293. https://pubmed.ncbi.nlm.nih.gov/37505311/
[7] Ma et al. (2022) Excessive intake of sugar: An accomplice of inflammation. Front Immunol Aug 31;13:988481. https://pubmed.ncbi.nlm.nih.gov/36119103/
[8] Pickard et al. (2017) Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev Sep;279(1):70-89. https://pubmed.ncbi.nlm.nih.gov/28856738/
[9] Twardowska et al. (2022) Preventing Bacterial Translocation in Patients with Leaky Gut Syndrome: Nutrition and Pharmacological Treatment Options. Int J Mol Sci Mar 16;23(6):3204. https://pubmed.ncbi.nlm.nih.gov/35328624/
[10] Wells et al. (2017) Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol Mar 1;312(3):G171-G193. https://pubmed.ncbi.nlm.nih.gov/27908847/